CRISPR-Edited Grass Carp Grow Faster via Disabling of Muscle Growth Brake.
GA, UNITED STATES, December 23, 2025 /EINPresswire.com/ -- This study shows that CRISPR-induced mutation of the mstnb gene in grass carp significantly accelerates growth. Edited fish were longer, heavier, and had thicker bodies than controls, mainly due to increased muscle fiber number rather than fiber size. The results demonstrate that targeting myostatin gene (mstnb) can effectively enhance muscle development and growth in aquaculture species.
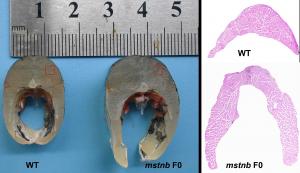
Researchers of Southwest University in Chongqing, China, have successfully used CRISPR/Cas9 gene-editing to make grass carp grow faster and develop more muscle by switching off a natural “brake” gene, mstnb. What’s new is that this boost in growth mainly comes from creating more muscle fibers, rather than simply enlarging existing ones—a finding that helps explain how muscle growth can be more precisely controlled in farmed fish.
Using CRISPR/Cas9, the research team edited the mstnb gene at the single-cell embryo stage. The edited fish grew significantly longer, heavier, and thicker than unedited fish within a few months. When examined for muscle tissues under a microscope, they found that the carp had denser muscle tissue made up of a much higher number of fibers, showing that the fish were building new muscle more actively.
“Mstnb acts as a key molecular brake on muscle formation in grass carp,” said corresponding Shengfei Dr Dai. “By removing this brake, we were able to unlock the fish’s natural capacity to produce more muscle cells and grow faster.”
This work improves on traditional selective breeding in aquaculture, which can take many generations to achieve similar gains. Instead of waiting for years, gene editing offers a way to make targeted, precise changes in a single generation. The findings also shed light on the role of myostatin-related genes in farmed fish, which were previously less well understood than in mammals.
One surprising result was that the muscle fibers themselves did not become much larger, instead, the fish built more fibers. “We expected the fibers to be thicker, but what we actually saw was a dramatic increase in fiber number,” Dai noted. “This suggests that different growth pathways can be selectively influenced through gene editing.”
While the results are promising for aquaculture, the researchers added that this is still early-stage work. Further studies are needed to test long-term health, stability across generations, and environmental safety before the approach could be used commercially.
DOI
10.1016/j.repbre.2025.09.001
Original Source URL
https://doi.org/10.1016/j.repbre.2025.09.001
Funding Information
This work was supported by Agriculture Biobreeding Major Project (2023ZD0405503) and Fundamental Research Funds for the Central Universities (SWU-XDJH202311 and SWU-KQ22061).
Lucy Wang
BioDesign Research
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.